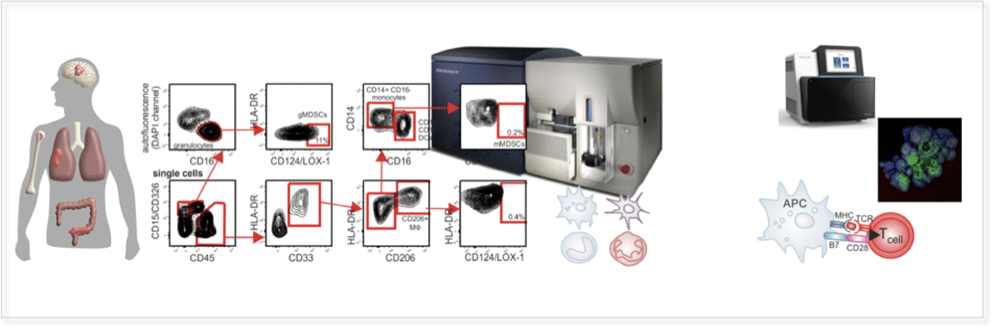
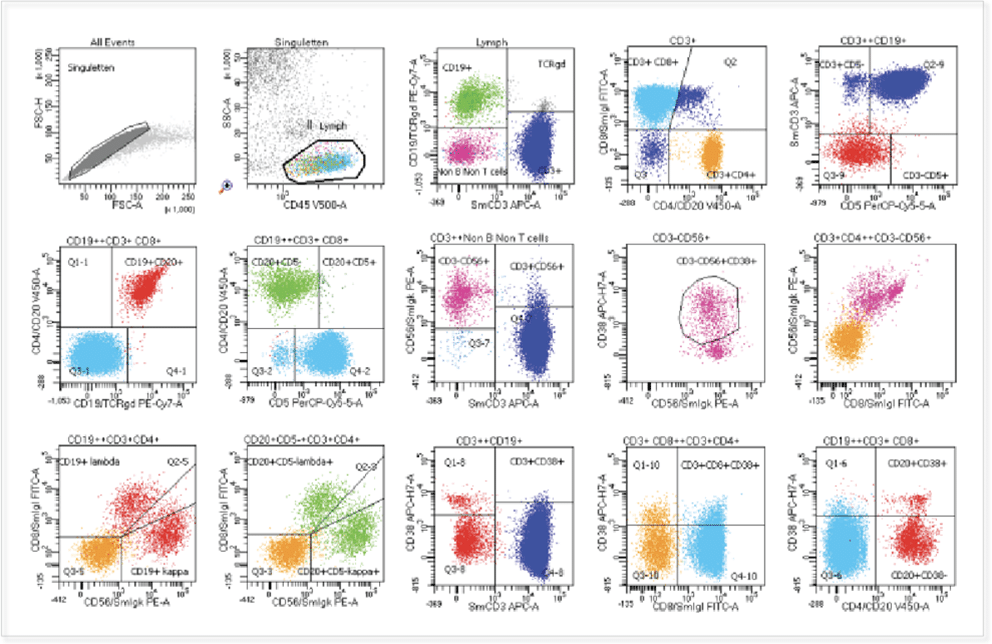
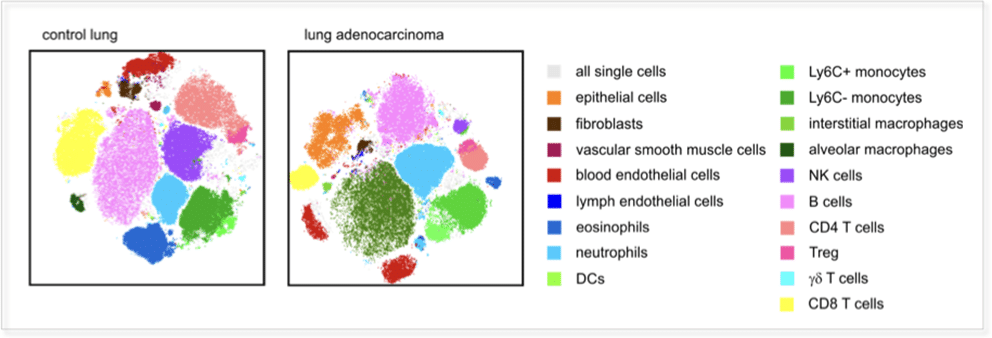
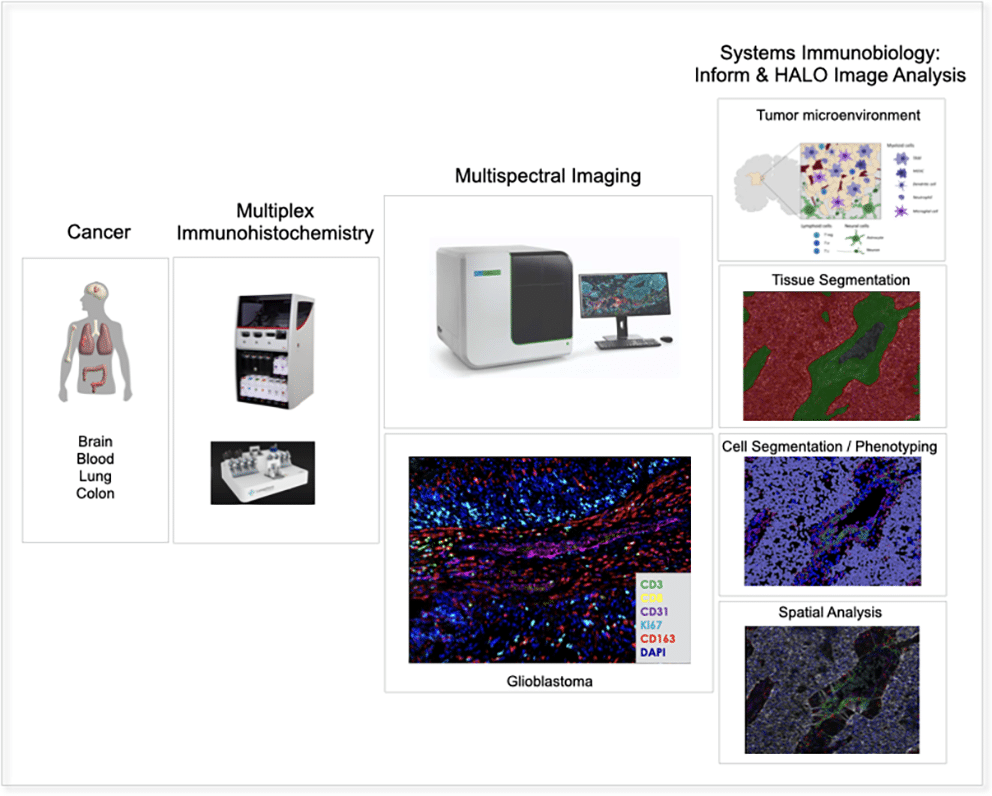
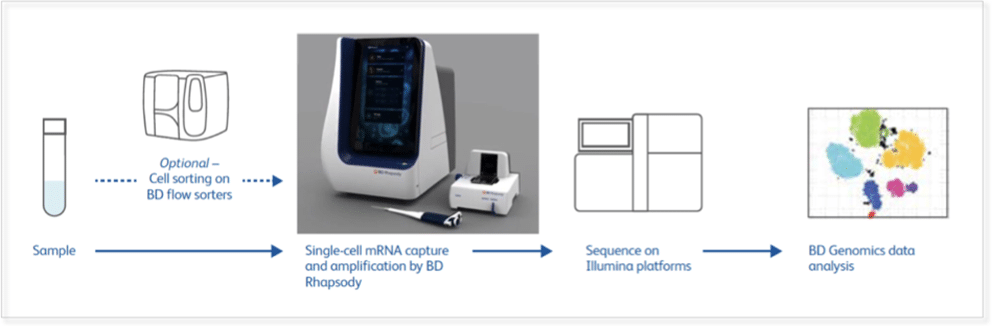
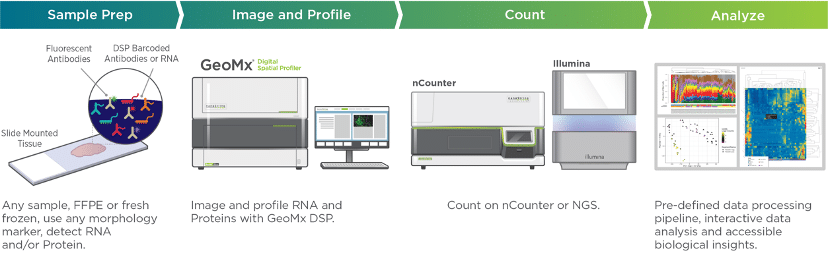
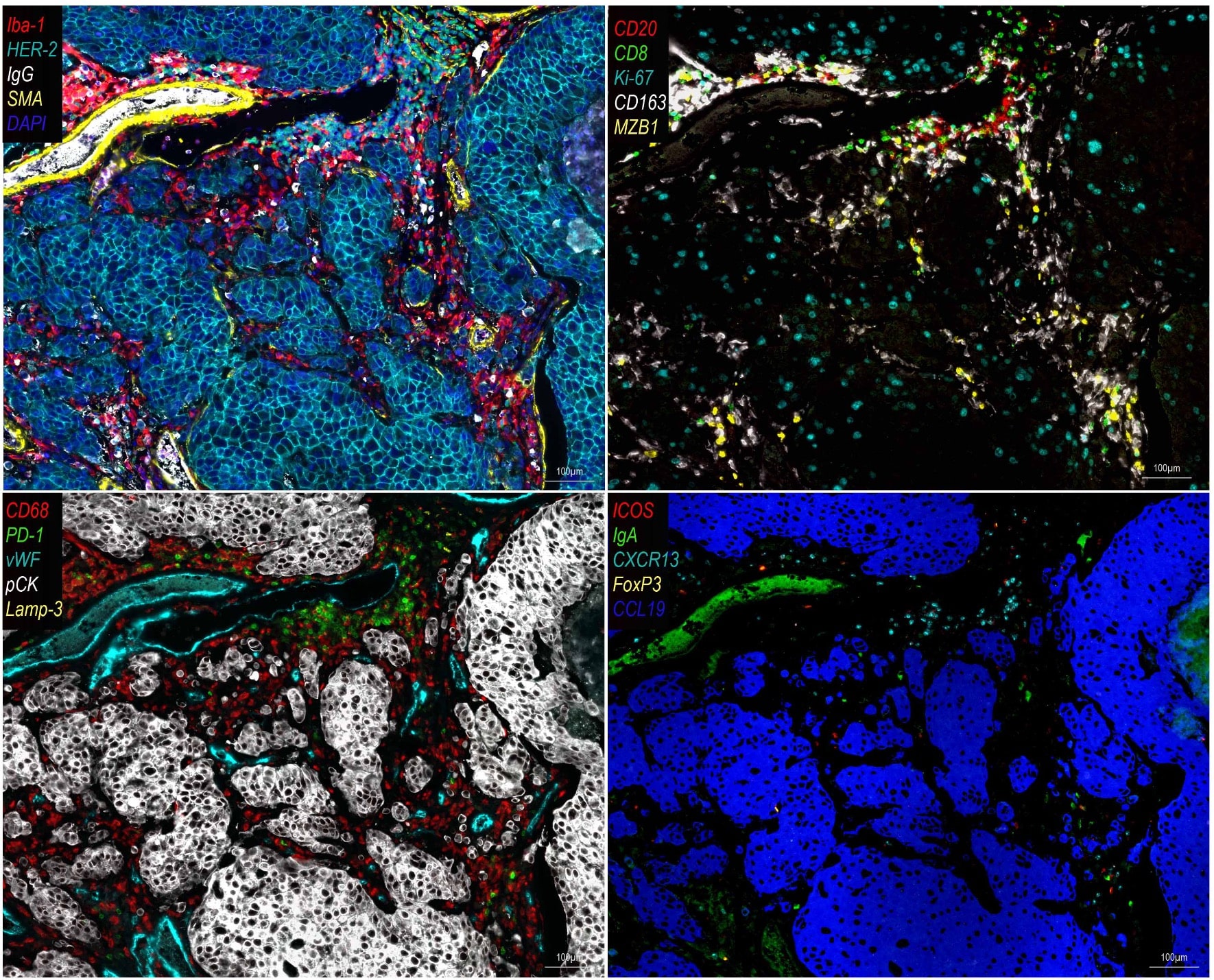
Focussed and clinically validated multi-color panels to investigate human and murine immune cells in each state of development
- Blood phenotyping
- T cell subsets
- B cell subsets
- NK cell subsets
- Lymphocyte differentation
- Lymphoid diseases
- Myeloid differentiation
- Myeloid diseases
- Monocyte subsets
- Immune checkpoints
- Stem / Progenitor cells
- Neurological diseases
- Neurovascular unit cells